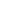D-SaLife, developed by DongWoon Anatech
based on advanced Semiconductor technology and know-how
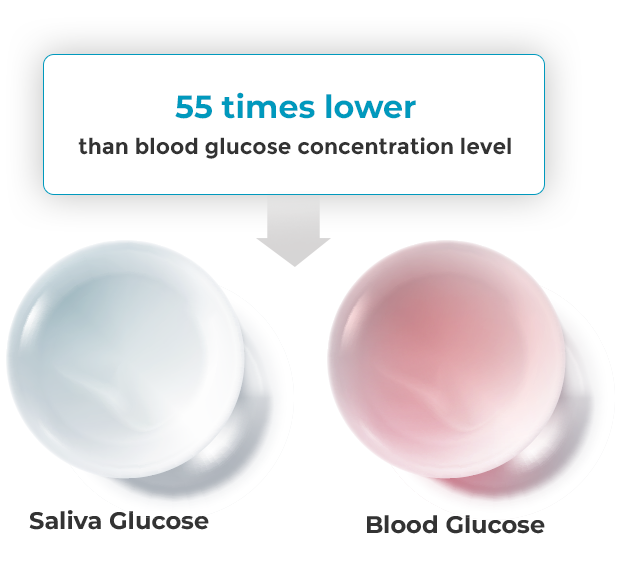
DongWoon Anatech has developed D-SaLife, the Saliva based Glucose measurement IVD device,
which adopts micro-electricity flow control technology that enables measurement, conversion
and output without loss of electricity, accumulated by system semiconductor design technology.
DongWoon Technology
-
AF
Autofocus driver chip on
Smartphone camerasGlobal No.1 M/S player in
Driver IC market (Released 1
Bil. of semiconductors in
2019) -
OIS
Driver chip to avoid hand
shaking on CameraHuawei smartphone adopted
-
HAPTIC
Chip for the reaction to touch
Smartphones and cars
adopted (Hyundai Genesis G*) -
ToF
3 D solution with Time of Flight
technology by using light emission
and reflectionSuccessful development of
Driver IC